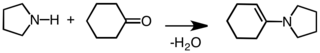Pyrrolidine
| |||
| Names | |||
|---|---|---|---|
Preferred IUPAC name Pyrrolidine | |||
| Other names Azolidine Azacyclopentane Tetrahydropyrrole Prolamine | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
ChEBI |
| ||
ChEMBL |
| ||
ChemSpider |
| ||
ECHA InfoCard | 100.004.227 | ||
PubChem CID |
| ||
RTECS number | UX9650000 | ||
UNII |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | C4H9N | ||
Molar mass | 71.12 g·mol−1 | ||
| Appearance | Clear colorless liquid | ||
Density | 0.866 g/cm3 | ||
Melting point | −63 °C (−81 °F; 210 K) | ||
Boiling point | 87 °C (189 °F; 360 K) | ||
Solubility in water | Miscible | ||
Acidity (pKa) | 11.27 (pKa of conjugate acid in water),[1] 19.56 (pKa of conjugate acid in acetonitrile)[2] | ||
Magnetic susceptibility (χ) | -54.8·10−6 cm3/mol | ||
| Hazards | |||
| Main hazards | highly flammable, harmful, corrosive, possible mutagen | ||
Safety data sheet | MSDS | ||
R-phrases (outdated) | R11-R20/21/22-R34[3] | ||
S-phrases (outdated) | S16-S26-S28-S36/37-S45 | ||
NFPA 704 |  3 3 1 | ||
Flash point | 3 °C (37 °F; 276 K) | ||
Autoignition temperature | 345 °C (653 °F; 618 K) | ||
| Related compounds | |||
Related nitrogen heterocyclic compounds | Pyrrole (aromatic with two double bonds) Pyrroline (one double bond) Pyrrolizidine (two pentagonal rings) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like".[4] In addition to pyrrolidine itself, many substituted pyrrolidines are known.
Contents
1 Synthesis and occurrence
2 Reactions
3 References
4 External links
Synthesis and occurrence
Pyrrolidine is produced by treatment of 1,4-butanediol with ammonia over an oxide catalyst.[5]
Many modifications of pyrrolidine are found in natural and synthetic chemistry. The pyrrolidine ring structure is present in numerous natural alkaloids such as nicotine and hygrine. It is found in many drugs such as procyclidine and bepridil. It also forms the basis for the racetam compounds (e.g. piracetam, aniracetam). The amino acids proline and hydroxyproline are, in a structural sense, derivatives of pyrrolidine.
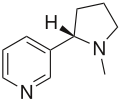
Nicotine contains an N-methylpyrrolidine ring linked to a pyridine ring.
Reactions
Pyrrolidine is a base. Its basicity is typical of other dialkyl amines.[6] Relative to many secondary amines, pyrrolidine is distinctive because of its compactness, a consequence of its cyclic structure.
Pyrrolidine is used as a building block in the synthesis of more complex organic compounds. It is used to activate ketones and aldehydes toward nucleophilic addition by formation of enamines:[7]
References
^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines". Journal of the American Chemical Society. 79 (20): 5441–5444. doi:10.1021/ja01577a030..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". The Journal of Organic Chemistry. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
^ MSDS
^ Pyrrolidine, The Good Scents Company
^ Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a02_001
^ H. K. Hall, Jr. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79 (20): 5441. doi:10.1021/ja01577a030.
^ R. B. Woodward, I. J. Pachter, and M. L. Scheinbaum (1974). "2,2-(Trimethylenedithio)cyclohexanone". Organic Syntheses. 54: 39.CS1 maint: Multiple names: authors list (link); Collective Volume, 6, p. 1014
External links
 Media related to Azolidines at Wikimedia Commons
Media related to Azolidines at Wikimedia Commons