Solvent

A bottle of acetic acid, a liquid solvent
A solvent (from the Latin solvō, "loosen, untie, solve") is a substance that dissolves a solute (a chemically distinct liquid, solid or gas), resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Common uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene), as paint thinners (e.g. toluene, turpentine), as nail polish removers and glue solvents (acetone, methyl acetate, ethyl acetate), in spot removers (e.g. hexane, petrol ether), in detergents (citrus terpenes) and in perfumes (ethanol). Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within a cell. Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syntheses and purification processes.
Contents
1 Solutions and solvation
2 Solvent classifications
2.1 Other polarity scales
2.2 Polar protic and polar aprotic
3 Multicomponent
3.1 Solvents
3.2 Thinners
4 Physical properties
4.1 Properties table of common solvents
4.1.1 Nonpolar solvents
4.1.2 Polar aprotic solvents
4.1.3 Polar protic solvents
4.2 Hansen solubility parameter values
4.2.1 Non-polar solvents
4.2.2 Polar aprotic solvents
4.2.3 Polar protic solvents
4.3 Boiling point
4.4 Density
5 Safety
5.1 Fire
5.2 Explosive peroxide formation
6 Health effects
6.1 Acute exposure
6.2 Chronic exposure
6.3 Environmental contamination
7 See also
8 References
9 Bibliography
10 External links
Solutions and solvation
When one substance is dissolved into another, a solution is formed.[1] This is opposed to the situation when the compounds are insoluble like sand in water. In a solution, all of the ingredients are uniformly distributed at a molecular level and no residue remains. A solvent-solute mixture consists of a single phase with all solute molecules occurring as solvates (solvent-solute complexes), as opposed to separate continuous phases as in suspensions, emulsions and other types of non-solution mixtures. The ability of one compound to be dissolved in another is known as solubility; if this occurs in all proportions, it is called miscible.
In addition to mixing, the substances in a solution interact with each other at the molecular level. When something is dissolved, molecules of the solvent arrange around molecules of the solute. Heat transfer is involved and entropy is increased making the solution more thermodynamically stable than the solute and solvent separately. This arrangement is mediated by the respective chemical properties of the solvent and solute, such as hydrogen bonding, dipole moment and polarizability.[2] Solvation does not cause a chemical reaction or chemical configuration changes in the solute. However, solvation resembles a coordination complex formation reaction, often with considerable energetics (heat of solvation and entropy of solvation) and is thus far from a neutral process.
Solvent classifications
Solvents can be broadly classified into two categories: polar and non-polar. A special case is mercury, whose solutions are known as amalgams; also, other metal solutions exist which are liquid at room temperature. Generally, the dielectric constant of the solvent provides a rough measure of a solvent's polarity. The strong polarity of water is indicated by its high dielectric constant of 88 (at 0 °C).[3] Solvents with a dielectric constant of less than 15 are generally considered to be nonpolar.[4] The dielectric constant measures the solvent's tendency to partly cancel the field strength of the electric field of a charged particle immersed in it. This reduction is then compared to the field strength of the charged particle in a vacuum.[4] Heuristically, the dielectric constant of a solvent can be thought of as its ability to reduce the solute's effective internal charge. Generally, the dielectric constant of a solvent is an acceptable predictor of the solvent's ability to dissolve common ionic compounds, such as salts.
Other polarity scales
Dielectric constants are not the only measure of polarity. Because solvents are used by chemists to carry out chemical reactions or observe chemical and biological phenomena, more specific measures of polarity are required. Most of these measures are sensitive to chemical structure.
The Grunwald–Winstein mY scale measures polarity in terms of solvent influence on buildup of positive charge of a solute during a chemical reaction.
Kosower's Z scale measures polarity in terms of the influence of the solvent on UV-absorption maxima of a salt, usually pyridinium iodide or the pyridinium zwitterion.[5]
Donor number and donor acceptor scale measures polarity in terms of how a solvent interacts with specific substances, like a strong Lewis acid or a strong Lewis base.[6]
The Hildebrand parameter is the square root of cohesive energy density. It can be used with nonpolar compounds, but cannot accommodate complex chemistry.
Reichardt's dye, a solvatochromic dye that changes color in response to polarity, gives a scale of ET(30) values. ET is the transition energy between the ground state and the lowest excited state in kcal/mol, and (30) identifies the dye. Another, roughly correlated scale (ET(33)) can be defined with Nile red.
The polarity, dipole moment, polarizability and hydrogen bonding of a solvent determines what type of compounds it is able to dissolve and with what other solvents or liquid compounds it is miscible. Generally, polar solvents dissolve polar compounds best and non-polar solvents dissolve non-polar compounds best: "like dissolves like". Strongly polar compounds like sugars (e.g. sucrose) or ionic compounds, like inorganic salts (e.g. table salt) dissolve only in very polar solvents like water, while strongly non-polar compounds like oils or waxes dissolve only in very non-polar organic solvents like hexane. Similarly, water and hexane (or vinegar and vegetable oil) are not miscible with each other and will quickly separate into two layers even after being shaken well.
Polarity can be separated to different contributions. For example, the Kamlet-Taft parameters are dipolarity/polarizability (π*), hydrogen-bonding acidity (α) and hydrogen-bonding basicity (β). These can be calculated from the wavelength shifts of 3–6 different solvatochromic dyes in the solvent, usually including Reichardt's dye, nitroaniline and diethylnitroaniline. Another option, Hansen's parameters, separate the cohesive energy density into dispersion, polar and hydrogen bonding contributions.
Polar protic and polar aprotic
Solvents with a dielectric constant (more accurately, relative static permittivity) greater than 15 (i.e. polar or polarizable) can be further divided into protic and aprotic. Protic solvents solvate anions (negatively charged solutes) strongly via hydrogen bonding. Water is a protic solvent. Aprotic solvents such as acetone or dichloromethane tend to have large dipole moments (separation of partial positive and partial negative charges within the same molecule) and solvate positively charged species via their negative dipole.[7] In chemical reactions the use of polar protic solvents favors the SN1 reaction mechanism, while polar aprotic solvents favor the SN2 reaction mechanism. These polar solvents are capable of forming hydrogen bonds with water to dissolve in water whereas non polar solvents are not capable of strong hydrogen bonds.
Multicomponent
It is the combination of substances that causes the large functionality of these products and their consumer properties.
Solvents
| Name | Composition |
|---|---|
| Solvent 645 | toluene 50%, butyl acetate 18%, ethyl acetate 12%, butanol 10%, ethanol 10%. |
| Solvent 646 | toluene 50%, ethanol 15%, butanol 10%, butyl- or amyl acetate 10%, ethyl cellosolve 8%, acetone 7%[8] |
| Solvent 647 | butyl- or amyl acetate 29.8%, ethyl acetate 21.2%, butanol 7.7%, toluene or pyrobenzene 41.3%[9] |
| Solvent 648 | butyl acetate 50%, ethanol 10%, butanol 20%, toluene 20%[10] |
| Solvent 649 | ethyl cellosolve 30%, butanol 20%, xylene 50% |
| Solvent 650 | ethyl cellosolve 20%, butanol 30%, xylene 50%[11] |
| Solvent 651 | white spirit 90%, butanol 10% |
| Solvent KR-36 | butyl acetate 20%, butanol 80% |
| Solvent P-4 | toluene 62%, acetone 26%, butyl acetate 12%. |
| Solvent P-10 | xylene 85%, acetone 15%. |
| Solvent P-12 | toluene 60%, butyl acetate 30%, xylene 10%. |
| Solvent P-14 | cyclohexanone 50%, toluene 50%. |
| Solvent P-24 | solvent 50%, xylene 35%, acetone 15%. |
| Solvent P-40 | toluene 50%, ethyl cellosolve 30%, acetone 20%. |
| Solvent P-219 | toluene 34%, cyclohexanone 33%, acetone 33%. |
| Solvent P-3160 | butanol 60%, ethanol 40%. |
| Solvent RCC | xylene 90%, butyl acetate 10%. |
| Solvent RML | ethanol 64%, ethylcellosolve 16%, toluene 10%, butanol 10%. |
| Solvent PML-315 | toluene 25%, xylene 25%, butyl acetate 18%, ethyl cellosolve 17%, butanol 15%. |
| Solvent PC-1 | toluene 60%, butyl acetate 30%, xylene 10%. |
| Solvent PC-2 | white spirit 70%, xylene 30%. |
| Solvent RFG | ethanol 75%, butanol 25%. |
| Solvent RE-1 | xylene 50%, acetone 20%, butanol 15%, ethanol 15%. |
| Solvent RE-2 | Solvent 70%, ethanol 20%, acetone 10%. |
| Solvent RE-3 | solvent 50%, ethanol 20%, acetone 20%, ethyl cellosolve 10%. |
| Solvent RE-4 | solvent 50%, acetone 30%, ethanol 20%. |
| Solvent FK-1 (?) | absolute alcohol (99.8%) 95%, ethyl acetate 5% |
Thinners
| Name | Composition |
|---|---|
| Thinner RKB-1 | butanol 50%, xylene 50% |
| Thinner RKB-2 | butanol 95%, xylene 5% |
| Thinner RKB-3 | xylene 90%, butanol 10% |
| Thinner M | ethanol 65%, butyl acetate 30%, ethyl acetate 5%. |
| Thinner P-7 | cyclohexanone 50%, ethanol 50%. |
| Thinner R-197 | xylene 60%, butyl acetate 20%, ethyl cellosolve 20%. |
| Thinner of WFD | toluene 50%, butyl acetate (or amyl acetate) 18%, butanol 10%, ethanol 10%, ethyl acetate 9%, acetone 3%. |
Physical properties
Properties table of common solvents
The solvents are grouped into nonpolar, polar aprotic, and polar protic solvents, with each group ordered by increasing polarity. The properties of solvents which exceed those of water are bolded.
| Solvent | Chemical formula | Boiling point[12] (°C) | Dielectric constant[13] | Density (g/mL) | Dipole moment (D) |
|---|---|---|---|---|---|
| |||||
Pentane | CH3CH2CH2CH2CH3 | 36 | 1.84 | 0.626 | 0.00 |
Cyclopentane | 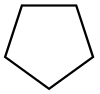 C5H10 | 40 | 1.97 | 0.751 | 0.00 |
Hexane | CH3CH2CH2CH2CH2CH3 | 69 | 1.88 | 0.655 | 0.00 |
Cyclohexane |  C6H12 | 81 | 2.02 | 0.779 | 0.00 |
Benzene | 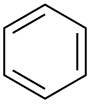 C6H6 | 80 | 2.3 | 0.879 | 0.00 |
Toluene | C6H5-CH3 | 111 | 2.38 | 0.867 | 0.36 |
1,4-Dioxane |  C4H8O2 | 101 | 2.3 | 1.033 | 0.45 |
Chloroform | CHCl3 | 61 | 4.81 | 1.498 | 1.04 |
Diethyl ether | CH3CH2-O-CH2CH3 | 35 | 4.3 | 0.713 | 1.15 |
Dichloromethane (DCM) | CH2Cl2 | 40 | 9.1 | 1.3266 | 1.60 |
| |||||
Tetrahydrofuran (THF) | 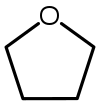 C4H8O | 66 | 7.5 | 0.886 | 1.75 |
Ethyl acetate |  CH3-C(=O)-O-CH2-CH3 | 77 | 6.02 | 0.894 | 1.78 |
Acetone |  CH3-C(=O)-CH3 | 56 | 21 | 0.786 | 2.88 |
Dimethylformamide (DMF) | 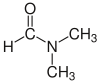 H-C(=O)N(CH3)2 | 153 | 38 | 0.944 | 3.82 |
Acetonitrile (MeCN) | CH3-C≡N | 82 | 37.5 | 0.786 | 3.92 |
Dimethyl sulfoxide (DMSO) |  CH3-S(=O)-CH3 | 189 | 46.7 | 1.092 | 3.96 |
Nitromethane | CH3-NO2 | 100–103 | 35.87 | 1.1371 | 3.56 |
Propylene carbonate | C4H6O3 | 240 | 64.0 | 1.205 | 4.9 |
| |||||
Formic acid |  H-C(=O)OH | 101 | 58 | 1.21 | 1.41 |
n-Butanol | CH3CH2CH2CH2OH | 118 | 18 | 0.810 | 1.63 |
Isopropyl alcohol (IPA) |  CH3-CH(-OH)-CH3 | 82 | 18 | 0.785 | 1.66 |
n-Propanol | CH3CH2CH2OH | 97 | 20 | 0.803 | 1.68 |
Ethanol | CH3CH2OH | 79 | 24.55 | 0.789 | 1.69 |
Methanol | CH3OH | 65 | 33 | 0.791 | 1.70 |
Acetic acid |  CH3-C(=O)OH | 118 | 6.2 | 1.049 | 1.74 |
Water | H-O-H | 100 | 80 | 1.000 | 1.85 |
Hansen solubility parameter values
The Hansen solubility parameter values[14][15] are based on dispersion bonds (δD), polar bonds (δP) and hydrogen bonds (δH). These contain information about the inter-molecular interactions with other solvents and also with polymers, pigments, nanoparticles, etc. This allows for rational formulations knowing, for example, that there is a good HSP match between a solvent and a polymer. Rational substitutions can also be made for "good" solvents (effective at dissolving the solute) that are "bad" (expensive or hazardous to health or the environment). The following table shows that the intuitions from "non-polar", "polar aprotic" and "polar protic" are put numerically – the "polar" molecules have higher levels of δP and the protic solvents have higher levels of δH. Because numerical values are used, comparisons can be made rationally by comparing numbers. For example, acetonitrile is much more polar than acetone but exhibits slightly less hydrogen bonding.
| Solvent | Chemical formula | δD Dispersion | δP Polar | δH Hydrogen bonding |
|---|---|---|---|---|
| ||||
Hexane | CH3CH2CH2CH2CH2CH3 | 14.9 | 0.0 | 0.0 |
Benzene | C6H6 | 18.4 | 0.0 | 2.0 |
Toluene | C6H5-CH3 | 18.0 | 1.4 | 2.0 |
Diethyl ether | CH3CH2-O-CH2CH3 | 14.5 | 2.9 | 4.6 |
Chloroform | CHCl3 | 17.8 | 3.1 | 5.7 |
1,4-Dioxane | /-CH2-CH2-O-CH2-CH2-O- | 17.5 | 1.8 | 9.0 |
| ||||
Ethyl acetate | CH3-C(=O)-O-CH2-CH3 | 15.8 | 5.3 | 7.2 |
Tetrahydrofuran (THF) | /-CH2-CH2-O-CH2-CH2- | 16.8 | 5.7 | 8.0 |
Dichloromethane | CH2Cl2 | 17.0 | 7.3 | 7.1 |
Acetone | CH3-C(=O)-CH3 | 15.5 | 10.4 | 7.0 |
Acetonitrile (MeCN) | CH3-C≡N | 15.3 | 18.0 | 6.1 |
Dimethylformamide (DMF) | H-C(=O)N(CH3)2 | 17.4 | 13.7 | 11.3 |
Dimethyl sulfoxide (DMSO) | CH3-S(=O)-CH3 | 18.4 | 16.4 | 10.2 |
| ||||
Acetic acid | CH3-C(=O)OH | 14.5 | 8.0 | 13.5 |
n-Butanol | CH3CH2CH2CH2OH | 16.0 | 5.7 | 15.8 |
Isopropanol | CH3-CH(-OH)-CH3 | 15.8 | 6.1 | 16.4 |
n-Propanol | CH3CH2CH2OH | 16.0 | 6.8 | 17.4 |
Ethanol | CH3CH2OH | 15.8 | 8.8 | 19.4 |
Methanol | CH3OH | 14.7 | 12.3 | 22.3 |
Formic acid | H-C(=O)OH | 14.6 | 10.0 | 14.0 |
Water | H-O-H | 15.5 | 16.0 | 42.3 |
If, for environmental or other reasons, a solvent or solvent blend is required to replace another of equivalent solvency, the substitution can be made on the basis of the Hansen solubility parameters of each. The values for mixtures are taken as the weighted averages of the values for the neat solvents. This can be calculated by trial-and-error, a spreadsheet of values, or HSP software.[14][15] A 1:1 mixture of toluene and 1,4 dioxane has δD, δP and δH values of 17.8, 1.6 and 5.5, comparable to those of chloroform at 17.8, 3.1 and 5.7 respectively. Because of the health hazards associated with toluene itself, other mixtures of solvents may be found using a full HSP dataset.
Boiling point
| Solvent | Boiling point (°C)[12] |
|---|---|
| ethylene dichloride | 83.48 |
| pyridine | 115.25 |
| methyl isobutyl ketone | 116.5 |
| methylene chloride | 39.75 |
| isooctane | 99.24 |
| carbon disulfide | 46.3 |
| carbon tetrachloride | 76.75 |
| o-xylene | 144.42 |
The boiling point is an important property because it determines the speed of evaporation. Small amounts of low-boiling-point solvents like diethyl ether, dichloromethane, or acetone will evaporate in seconds at room temperature, while high-boiling-point solvents like water or dimethyl sulfoxide need higher temperatures, an air flow, or the application of vacuum for fast evaporation.
- Low boilers: boiling point below 100 °C (boiling point of water)
- Medium boilers: between 100 °C and 150 °C
- High boilers: above 150 °C
Density
Most organic solvents have a lower density than water, which means they are lighter than and will form a layer on top of water. Important exceptions are most of the halogenated solvents like dichloromethane or chloroform will sink to the bottom of a container, leaving water as the top layer. This is crucial to remember when partitioning compounds between solvents and water in a separatory funnel during chemical syntheses.
Often, specific gravity is cited in place of density. Specific gravity is defined as the density of the solvent divided by the density of water at the same temperature. As such, specific gravity is a unitless value. It readily communicates whether a water-insoluble solvent will float (SG < 1.0) or sink (SG > 1.0) when mixed with water.
| Solvent | Specific gravity[16] |
|---|---|
| Pentane | 0.626 |
| Petroleum ether | 0.656 |
| Hexane | 0.659 |
| Heptane | 0.684 |
| Diethyl amine | 0.707 |
| Diethyl ether | 0.713 |
| Triethyl amine | 0.728 |
| Tert-butyl methyl ether | 0.741 |
| Cyclohexane | 0.779 |
| Tert-butyl alcohol | 0.781 |
| Isopropanol | 0.785 |
| Acetonitrile | 0.786 |
| Ethanol | 0.789 |
| Acetone | 0.790 |
| Methanol | 0.791 |
| Methyl isobutyl ketone | 0.798 |
| Isobutyl alcohol | 0.802 |
| 1-Propanol | 0.803 |
| Methyl ethyl ketone | 0.805 |
| 2-Butanol | 0.808 |
| Isoamyl alcohol | 0.809 |
| 1-Butanol | 0.810 |
| Diethyl ketone | 0.814 |
| 1-Octanol | 0.826 |
| p-Xylene | 0.861 |
| m-Xylene | 0.864 |
| Toluene | 0.867 |
| Dimethoxyethane | 0.868 |
| Benzene | 0.879 |
| Butyl acetate | 0.882 |
| 1-Chlorobutane | 0.886 |
| Tetrahydrofuran | 0.889 |
| Ethyl acetate | 0.895 |
| o-Xylene | 0.897 |
| Hexamethylphosphorus triamide | 0.898 |
| 2-Ethoxyethyl ether | 0.909 |
| N,N-Dimethylacetamide | 0.937 |
Diethylene glycol dimethyl ether | 0.943 |
| N,N-Dimethylformamide | 0.944 |
| 2-Methoxyethanol | 0.965 |
| Pyridine | 0.982 |
| Propanoic acid | 0.993 |
| Water | 1.000 |
| 2-Methoxyethyl acetate | 1.009 |
| Benzonitrile | 1.01 |
| 1-Methyl-2-pyrrolidinone | 1.028 |
| Hexamethylphosphoramide | 1.03 |
| 1,4-Dioxane | 1.033 |
| Acetic acid | 1.049 |
| Acetic anhydride | 1.08 |
| Dimethyl sulfoxide | 1.092 |
| Chlorobenzene | 1.1066 |
| Deuterium oxide | 1.107 |
| Ethylene glycol | 1.115 |
| Diethylene glycol | 1.118 |
| Propylene carbonate | 1.21 |
| Formic acid | 1.22 |
| 1,2-Dichloroethane | 1.245 |
| Glycerin | 1.261 |
| Carbon disulfide | 1.263 |
| 1,2-Dichlorobenzene | 1.306 |
| Methylene chloride | 1.325 |
| Nitromethane | 1.382 |
| 2,2,2-Trifluoroethanol | 1.393 |
| Chloroform | 1.498 |
| 1,1,2-Trichlorotrifluoroethane | 1.575 |
| Carbon tetrachloride | 1.594 |
| Tetrachloroethylene | 1.623 |
Safety
Fire
Most organic solvents are flammable or highly flammable, depending on their volatility. Exceptions are some chlorinated solvents like dichloromethane and chloroform. Mixtures of solvent vapors and air can explode. Solvent vapors are heavier than air; they will sink to the bottom and can travel large distances nearly undiluted. Solvent vapors can also be found in supposedly empty drums and cans, posing a flash fire hazard; hence empty containers of volatile solvents should be stored open and upside down.
Both diethyl ether and carbon disulfide have exceptionally low autoignition temperatures which increase greatly the fire risk associated with these solvents. The autoignition temperature of carbon disulfide is below 100 °C (212 °F), so objects such as steam pipes, light bulbs, hotplates, and recently-extinguished bunsen burners are able to ignite its vapours.
In addition some solvents, such as methanol, can burn with a very hot flame which can be nearly invisible under some lighting conditions.[17][18] This can delay or prevent the timely recognition of a dangerous fire, until flames spread to other materials.
Explosive peroxide formation
Ethers like diethyl ether and tetrahydrofuran (THF) can form highly explosive organic peroxides upon exposure to oxygen and light. THF is normally more likely to form such peroxides than diethyl ether. One of the most susceptible solvents is diisopropyl ether, but all ethers are considered to be potential peroxide sources.
The heteroatom (oxygen) stabilizes the formation of a free radical which is formed by the abstraction of a hydrogen atom by another free radical.[clarification needed] The carbon-centred free radical thus formed is able to react with an oxygen molecule to form a peroxide compound. The process of peroxide formation is greatly accelerated by exposure to even low levels of light, but can proceed slowly even in dark conditions.
Unless a desiccant is used which can destroy the peroxides, they will concentrate during distillation, due to their higher boiling point. When sufficient peroxides have formed, they can form a crystalline, shock-sensitive solid precipitate at the mouth of a container or bottle. Minor mechanical disturbances, such as scraping the inside of a vessel or the dislodging of a deposit, merely twisting the cap may provide sufficient energy for the peroxide to explode or detonate. Peroxide formation is not a significant problem when fresh solvents are used up quickly; they are more of a problem in laboratories which may take years to finish a single bottle. Low-volume users should acquire only small amounts of peroxide-prone solvents, and dispose of old solvents on a regular periodic schedule.
To avoid explosive peroxide formation, ethers should be stored in an aritight container, away from light, because both light and air can encourage peroxide formation.[19]
A number of tests can be used to detect the presence of a peroxide in an ether; one is to use a combination of iron(II) sulfate and potassium thiocyanate. The peroxide is able to oxidize the Fe2+ ion to an Fe3+ ion, which then forms a deep-red coordination complex with the thiocyanate.
Peroxides may be removed by washing with acidic iron(II) sulfate, filtering through alumina, or distilling from sodium/benzophenone. Aluminum does not destroy the peroxides but merely traps them, and must be disposed of properly. The advantage of using sodium/benzophenone is that moisture and oxygen are removed as well.[citation needed]
Health effects
General health hazards associated with solvent exposure include toxicity to the nervous system, reproductive damage, liver and kidney damage, respiratory impairment, cancer, and dermatitis.[20]
Acute exposure
Many solvents can lead to a sudden loss of consciousness if inhaled in large amounts. Solvents like diethyl ether and chloroform have been used in medicine as anesthetics, sedatives, and hypnotics for a long time. Ethanol (grain alcohol) is a widely used and abused psychoactive drug. Diethyl ether, chloroform, and many other solvents e.g. from gasoline or glues are abused recreationally in glue sniffing, often with harmful long term health effects like neurotoxicity or cancer.
Fraudulent substitution of 1,5-pentanediol for the psychoactive 1,4-butanediol by a subcontractor caused the Bindeez product recall.[21]
If ingested, the so called toxic alcohols (other than ethanol) such as methanol, propanol, and ethylene glycol metabolize into toxic aldehydes and acids, which cause potentially fatal metabolic acidosis.[22] The commonly available alcohol solvent methanol can cause permanent blindness or death if ingested. The solvent 2-butoxyethanol, used in fracking fluids, can cause hypotension and metabolic acidosis.[23]
Chronic exposure
Some solvents including chloroform and benzene a common ingredient in gasoline are known to be carcinogenic, while many others are considered by the World Health Organization to be likely carcinogens. Solvents can damage internal organs like the liver, the kidneys, the nervous system, or the brain. The cumulative effects of long-term or repeated exposure to solvents are called chronic solvent-induced encephalopathy (CSE).
Chronic exposure to organic solvents in the work environment can produce a range of adverse neuropsychiatric effects. For example, occupational exposure to organic solvents has been associated with higher numbers of painters suffering from alcoholism.[24] Ethanol has a synergistic effect when taken in combination with many solvents; for instance, a combination of toluene/benzene and ethanol causes greater nausea/vomiting than either substance alone.
Many solvents are known or suspected to be cataractogenic, greatly increasing the risk of developing cataracts in the lens of the eye.[25] Solvent exposure has also been associated with neurotoxic damage causing hearing loss[26][27] and color vision losses.[28]
Environmental contamination
A major pathway to induce health effects arises from spills or leaks of solvents that reach the underlying soil. Since solvents readily migrate substantial distances, the creation of widespread soil contamination is not uncommon; this is particularly a health risk if aquifers are affected. There may be about 5000 sites worldwide that have major subsurface solvent contamination.[citation needed]
See also
| Wikimedia Commons has media related to Solvents. |
- Free energy of solvation
- Solvents are often refluxed with an appropriate desiccant prior to distillation to remove water. This may be performed prior to a chemical synthesis where water may interfere with the intended reaction
- List of water-miscible solvents
- Lyoluminescence
- Occupational health
Partition coefficient (log P) is a measure of differential solubility of a compound in two solvents- Solvation
- Solution
- Solvent systems exist outside the realm of ordinary organic solvents: Supercritical fluids, ionic liquids and deep eutectic solvents
- Water model
- Water pollution
References
^ Tinoco, Ignacio; Sauer, Kenneth and Wang, James C. (2002) Physical Chemistry Prentice Hall p. 134 .mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
ISBN 0-13-026607-8
^ Lowery and Richardson, pp. 181–183
^ Malmberg, C. G.; Maryott, A. A. (January 1956). "Dielectric Constant of Water from 0° to 100°C" (PDF). Journal of Research of the National Bureau of Standards. 56 (1): 1. doi:10.6028/jres.056.001. Archived (PDF) from the original on 19 August 2014. Retrieved 27 June 2014.
^ ab Lowery and Richardson, p. 177.
^ Kosower, E.M. (1969) "An introduction to Physical Organic Chemistry" Wiley: New York, p. 293
^ Gutmann, V. (1976). "Solvent effects on the reactivities of organometallic compounds". Coord. Chem. Rev. 18 (2): 225. doi:10.1016/S0010-8545(00)82045-7.
^ Lowery and Richardson, p. 183.
^ dcpt.ru Solvent 646 Characteristics (ru)
^ dcpt.ru Solvent 647 Characteristics (ru)
^ dcpt.ru Solvent 648 Characteristics (ru)
^ dcpt.ru Solvent 650 Characteristics (ru)
^ ab Solvent Properties – Boiling Point Archived 14 June 2011 at the Wayback Machine.. Xydatasource.com. Retrieved on 2013-01-26.
^ Dielectric Constant Archived 4 July 2010 at the Wayback Machine.. Macro.lsu.edu. Retrieved on 2013-01-26.
^ ab Abbott, Steven and Hansen, Charles M. (2008) Hansen Hansen Solubility Parameters in Practice Archived 29 July 2016 at the Wayback Machine.,
ISBN 0-9551220-2-3
^ ab Hansen, Charles M. (2007) Hansen solubility parameters: a user's handbook Archived 25 June 2016 at the Wayback Machine. CRC Press,
ISBN 0-8493-7248-8
^ Selected solvent properties – Specific Gravity Archived 14 June 2011 at the Wayback Machine.. Xydatasource.com. Retrieved on 2013-01-26.
^ Fanick, E. Robert; Smith, Lawrence R.; Baines, Thomas M. (1 October 1984). "Safety Related Additives for Methanol Fuel". Warrendale, PA. Archived from the original on 12 August 2017.
^ Anderson, J. E.; Magyarl, M. W.; Siegl, W. O. (1985-07-01). "Concerning the Luminosity of Methanol-Hydrocarbon Diffusion Flames". Combustion Science and Technology. 43 (3–4): 115–125. doi:10.1080/00102208508947000. ISSN 0010-2202.
^ "Peroxides and Ethers | Environmental Health, Safety and Risk Management". www.uaf.edu. Retrieved 2018-01-25.
^ U.S. Department of Labor > Occupational Safety & Health Administration > Solvents Archived 15 March 2016 at the Wayback Machine.. osha.gov
^ "Recall ordered for toy that turns into drug - National - theage.com.au". www.theage.com.au. Retrieved 2018-07-01.
^ J.A. Kraut, M.E. Mullins Toxic Alcohols - Review N Engl J Med 2018;378:270-280(subscription required)
^ Hung, Tawny; Dewitt, Christopher R.; Martz, Walter; Schreiber, William; Holmes, Daniel Thomas (July 2010). "Fomepizole fails to prevent progression of acidosis in 2-Butoxyethanol and ethanol coingestion". Clinical Toxicology. 48 (6): 569–571. doi:10.3109/15563650.2010.492350. PMID 20560787.
^ Lundberg I, Gustavsson A, Högberg M, Nise G (1992). "Diagnoses of alcohol abuse and other neuropsychiatric disorders among house painters compared with house carpenters". Br J Ind Med. 49 (6): 409–15. doi:10.1136/oem.49.6.409. PMC 1012122. PMID 1606027.
^ Raitta, C; Husman, K; Tossavainen, A (1976). "Lens changes in car painters exposed to a mixture of organic solvents". Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie. 200 (2): 149–56. doi:10.1007/bf00414364. PMID 1086605.
^ Campo, Pierre; Morata, Thais C.; Hong, OiSaeng. "Chemical exposure and hearing loss". Disease-a-Month. 59 (4): 119–138. doi:10.1016/j.disamonth.2013.01.003. PMC 4693596. PMID 23507352. Archived from the original on 21 December 2017.
^ Johnson AC and Morata,, TC (2010). "Occupational exposure to chemicals and hearing impairment. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals" (PDF). Arbete och Hälsa. 44: 177. Archived (PDF) from the original on 4 June 2016.
^ Mergler, D; Blain, L; Lagacé, J. P. (1987). "Solvent related colour vision loss: An indicator of neural damage?". International Archives of Occupational and Environmental Health. 59 (4): 313–21. doi:10.1007/bf00405275. PMID 3497110.
Bibliography
- Lowery, T.H. and Richardson, K.S., Mechanism and Theory in Organic Chemistry, Harper Collins Publishers 3rd ed. 1987
ISBN 0-06-364044-9
External links
| Look up solvent in Wiktionary, the free dictionary. |
"European Solvents Industry Group - ESIG - ESIG European Solvents Industry Group" Solvents in Europe.
Table and text O-Chem Lecture
Tables Properties and toxicities of organic solvents- CDC – Organic Solvents – NIOSH Workplace Safety and Health Topic
- EPA - Solvent Contaminated Wipes