Dehydroalanine
 | |
 | |
| Names | |
|---|---|
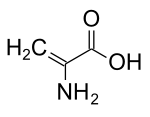
IUPAC name 2-Aminoprop-2-enoic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
DrugBank |
|
KEGG |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C3H5NO2 |
Molar mass | 87.08 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It exists both in its free form, produced by some pyridoxal 5'-phosphate-dependent α,β-eliminases[1], and as a residue found in peptides of microbial origin. It is unusual in having an unsaturated backbone.[2]
Structure and reactivity
As a primary enamine, dehydroalanine is unstable with respect to tautomerization. It therefore does not exist in large amounts or as a stable pure substance. However, N-acylated derivatives, such as peptides and related compounds, are stable.
Despite its name, dehydroalanine is not derived biochemically from alanine. Instead, it arises via a post translational modification of serine or cysteine. These amino acids, as residues in peptide chains, undergo enzyme-mediated loss of water and hydrogen sulfide, respectively.
Most amino acid residues are unreactive toward nucleophiles, but those containing dehydroalanine or some other dehydroamino acids are exceptions. These are electrophilic due to the α,β-unsaturated carbonyl,[2] and can, for example, alkylate other amino acids. This activity has made DHA useful synthetically to prepare lanthionine.
Occurrence
The dehydroalanine residue was first detected in nisin, a cyclic peptide with antimicrobial activity.[2] Dehydroalanine is also present in some lantibiotics and microcystins.
DHA can be formed from cysteine or serine by simple base catalysis without the need for an enzyme, which can happen during cooking and alkaline food preparation processes. It can then alkylate other amino acid residues, such as lysine, forming lysinoalanine cross-links and racemization of the original alanine. The resulting proteins are have lower nutritional quality for some species but higher nutritional quality for others. Some lysinoalanines may also cause kidney enlargement in rats.[3]
Many dehydroalanine-containing peptides are toxic.[citation needed]
A dehydroalanine residue was long thought to be an important electrophilic catalytic residue in histidine ammonia-lyase and phenylalanine ammonia-lyase enzymes, but the active residue was later found instead to be a different unsaturated alanine derivative—3,5-dihydro-5-methyldiene-4H-imidazol-4-one—that is even more electrophilic.[4][5]
References
^ Downs, DM; Ernst, DC (April 2015). "From microbiology to cancer biology: the Rid protein family prevents cellular damage caused by endogenously generated reactive nitrogen species". Molecular microbiology. 96 (2): 211–9. doi:10.1111/mmi.12945. PMID 25620221..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abc Siodłak, Dawid (2015). "α,β-Dehydroamino Acids in Naturally Occurring Peptides". Amino Acids. 47: 1–17. doi:10.1007/s00726-014-1846-4. PMC 4282715.
^ Friedman, Mendel (1999). "Lysinoalanine in food and in antimicrobial proteins". In Jackson, Lauren S.; Knize, Mark G.; Morgan, Jeffrey N. Impact of Processing on Food Safety. 459. Springer. pp. 145–159. doi:10.1007/978-1-4615-4853-9_10. ISBN 978-1-4615-4853-9. PMID 10335374.
^ Rétey, János (2003). "Discovery and role of methylidene imidazolone, a highly electrophilic prosthetic group". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1647 (1–2): 179–184. doi:10.1016/S1570-9639(03)00091-8.
^ Calabrese JC, Jordan DB, Boodhoo A, Sariaslani S, Vannelli T (September 2004). "Crystal structure of phenylalanine ammonia lyase: multiple helix dipoles implicated in catalysis". Biochemistry. 43 (36): 11403–16. doi:10.1021/bi049053+. PMID 15350127.