Arabinose
 | |
| Names | |
|---|---|
IUPAC name Arabinose | |
| Other names Pectinose | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
ECHA InfoCard | 100.005.182 |
EC Number | 205-699-8 |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties[1] | |
Chemical formula | C5H10O5 |
Molar mass | 150.13 g/mol |
| Appearance | Colorless crystals as prisms or needles |
Density | 1.585 g/cm3 (20 ºC) |
Melting point | 164 to 165 °C (327 to 329 °F; 437 to 438 K) |
Solubility in water | 834 g/1 L (25 °C (77 °F)) |
Magnetic susceptibility (χ) | -85.70·10−6 cm3/mol |
| Hazards | |
NFPA 704 |  1 1 0 |
| Related compounds | |
Related aldopentoses | Ribose Xylose Lyxose |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde (CHO) functional group.
For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, or structurally analogous to D-glyceraldehyde.[note 1] However, L-arabinose is in fact more common than D-arabinose in nature and is found in nature as a component of biopolymers such as hemicellulose and pectin.[2]
The L-arabinose operon, also known as the araBAD operon, has been the subject of much biomolecular research. The operon directs the catabolism of arabinose in E. coli, and it is dynamically activated in the presence of arabinose and the absence of glucose.[3]
A classic method for the organic synthesis of arabinose from glucose is the Wohl degradation.[4]
D-Arabinose
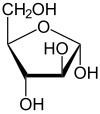
α-D-Arabinofuranose
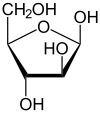
β-D-Arabinofuranose
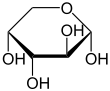
α-D-Arabinopyranose
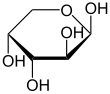
β-D-Arabinopyranose
Contents
1 Etymology
2 Use in biology
3 Use in foods
4 See also
5 Notes
6 References
Etymology
Arabinose gets its name from gum arabic, from which it was first isolated.[5]
Use in biology
In synthetic biology, arabinose is often used as a one-way or reversible switch for protein expression under the Pbad promoter in E. coli. This on-switch can be negated by the presence of glucose or reversed off by the addition of glucose in the culture medium which is a form of catabolite repression.[6]
Some organic acid tests check for the presence of arabinose, which may indicate overgrowth of intestinal yeast such as Candida albicans or other yeast/fungus species.[citation needed]
Use in foods
Originally commercialized as a sweetener, arabinose is an inhibitor of sucrase, the enzyme that breaks down sucrose into glucose and fructose in the small intestine.[7] This inhibitory effect has been validated both in rodents and humans.[7][8] Therefore, arabinose could be used in foods to attenuate the peak of glycemic response (see: glycemic index) after the consumption of sucrose. The long-term effects of arabinose consumption on blood glucose parameters such as HbA1c and fasting blood glucose levels are unknown. Foods that contain arabinose are usually designed for prediabetic and diabetic patients. These foods are especially popular in Japan and China, where arabinose is legally used as a food additive.
Arabinose is a potential prebiotic, because it cannot be absorbed by human intestine and could be utilized by probiotics such as bifidobacteria.[9] This claim requires further validation.
See also
- Arabinosyl nucleosides
Notes
^ For sugars, the D/L nomenclature does not refer to the molecule's optical rotation properties but to its structural analogy to glyceraldehyde.
References
^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-110. ISBN 0-8493-0462-8..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Holtzapple, M.T. (2003). HEMICELLULOSES. Encyclopedia of Food Sciences and Nutrition. pp. 3060–3071. doi:10.1016/B0-12-227055-X/00589-7. ISBN 9780122270550.
^ Watson, James (2003). Molecular Biology of the Gene. p. 503.
^ Braun, Géza (1940). "D-Arabinose". Organic Syntheses. 20: 14.; Collective Volume, 3, p. 101
^ Merriam Webster Dictionary
^ Guzman LM, Belin D, Carson MJ, Beckwith J (July 1995). "Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter". J. Bacteriol. 177 (14): 4121–30. CiteSeerX 10.1.1.629.9409. doi:10.1128/jb.177.14.4121-4130.1995. PMC 177145. PMID 7608087.
^ ab Krog-Mikkelsen, Inger; Hels, Ole; Tetens, Inge; Holst, Jens Juul; Andersen, Jens Rikardt; Bukhave, Klaus (2011-08-01). "The effects of L-arabinose on intestinal sucrase activity: dose-response studies in vitro and in humans". The American Journal of Clinical Nutrition. 94 (2): 472–478. doi:10.3945/ajcn.111.014225. ISSN 1938-3207. PMID 21677059.
^ Seri, K.; Sanai, K.; Matsuo, N.; Kawakubo, K.; Xue, C.; Inoue, S. (1996-11-01). "L-arabinose selectively inhibits intestinal sucrase in an uncompetitive manner and suppresses glycemic response after sucrose ingestion in animals". Metabolism: Clinical and Experimental. 45 (11): 1368–1374. doi:10.1016/s0026-0495(96)90117-1. ISSN 0026-0495. PMID 8931641.
^ Degnan, B. A.; Macfarlane, G. T. (1993). "Transport and metabolism of glucose and arabinose in Bifidobacterium breve". Archives of Microbiology. 160 (2): 144–151. doi:10.1007/BF00288717. ISSN 0302-8933.



